Accelerating Clinical Data Review: Addressing Fragmentation, Improving Collaboration, and Reducing Review Cycle Times by 35%
Download PDF: Phastar-Beaconcure Case Study
Industry Challenge: Disconnected Review Workflows, Lack of Automation, and Lengthy Data Review Cycles
Clinical data analysis review remains a critical, yet often fragmented, element of the clinical trial process. Many organizations still rely on manual workflows, using email or instant messaging-based feedback loops with marked up spreadsheets in disconnected documentation systems. These traditional approaches can slow down review continuity, increase the risk of errors, and create barriers to clear oversight, particularly in large, multi-stakeholder clinical trials with significant reporting efforts.
Persistent challenges across the industry include:
- Limited visibility into ongoing review activities
- Sequential actions that prolong the turnaround time
- Difficulty maintaining traceability of feedback, decisions, and data changes
- Manual reconciliation of comments and annotations across disparate systems or files
- Rework cycles caused by workflow discontinuities
- Poor documentation of review history when team members or roles shift
- Lack of transparency in cross-company collaborations
These inefficiencies ultimately delay submission timelines and reduce the clarity sponsors need to make informed decisions—slowing the progress of promising treatments.
As the demand for faster, more transparent, and audit-ready clinical reporting operations grows, sponsors are reevaluating how to structure their review processes and CRO interactions. An ongoing collaboration between Beaconcure and Phastar reflects this broader shift to help address these challenges: combining cutting-edge technology with specialist biometrics and data science expertise to rethink how data reporting oversight, documentation review, and communication are handled across clinical trials and across companies.
Solution:
A Centralized Platform for Seamless Data Analysis Review Management
To address these operational inefficiencies and enable real-time visibility, Beaconcure developed Verify, a centralized platform designed to streamline the review and validation of clinical trial summary tables, listings, and figures (TLFs). The platform provides a unified, structured environment for reviewing outputs and reference documents, improving collaboration, traceability, and overall quality. It is also designed to shorten review times, ultimately accelerating time to submission.
Phastar, a specialist CRO focused on biometrics and data science, partnered with Beaconcure to implement and configure Verify for Phastar’s clinical data analyses, TLF reviews, and downstream automations. The goal: to reduce cycle times, improve sponsor visibility, minimize required sponsor effort, and establish cross-company workflow automations for Phastar clients who experience pain points in the current TLF review and post hoc analysis creation cycles.
Features that Deliver the Difference
Live Output Interaction
Sponsor reviewers can view parsed TLFs and reference documents directly in Verify, eliminating the inefficiencies of static file reviews and enabling immediate feedback.
Centralized Feedback and Context Preservation
All comments, annotations, and decisions are tied to specific outputs—preserving reviewer rationale and ensuring a complete, auditable history of changes, even as team members rotate or project phases shift.
Version Tracking and Traceability
File versions, reviewer actions, and comments are automatically logged— making the process traceable and inspection-ready for future regulatory audits.
Layered Workflow Views
Verify provides role-based workflow views for different stakeholders. Whether tracking QC progress, managing file versions, or conducting task-based reviews, each user has a clear view of responsibilities—driving accountability and reducing review cycles.
Role-Based Dashboards
Sponsor and internal stakeholders have customized visibility of outstanding tasks, deadlines, and review status—allowing them to focus on what matters most, consolidate feedback, and cut unnecessary follow-ups.
Outcome:
Improved Visibility, Over 35% Faster Review Cycles, and Reduced Risk of Rework
Centralizing activities within one platform helps complete tasks faster with fewer iterations, reducing the risk of missed or delayed actions and supporting earlier study milestones. By embedding Verify into core workflows, Phastar has laid the groundwork for faster, more efficient, and fully documented data reporting review—supporting the broader goal of accelerating clinical development timelines and empowering sponsors with the visibility and agility needed to navigate increasingly complex trials.
Key Benefits Delivered
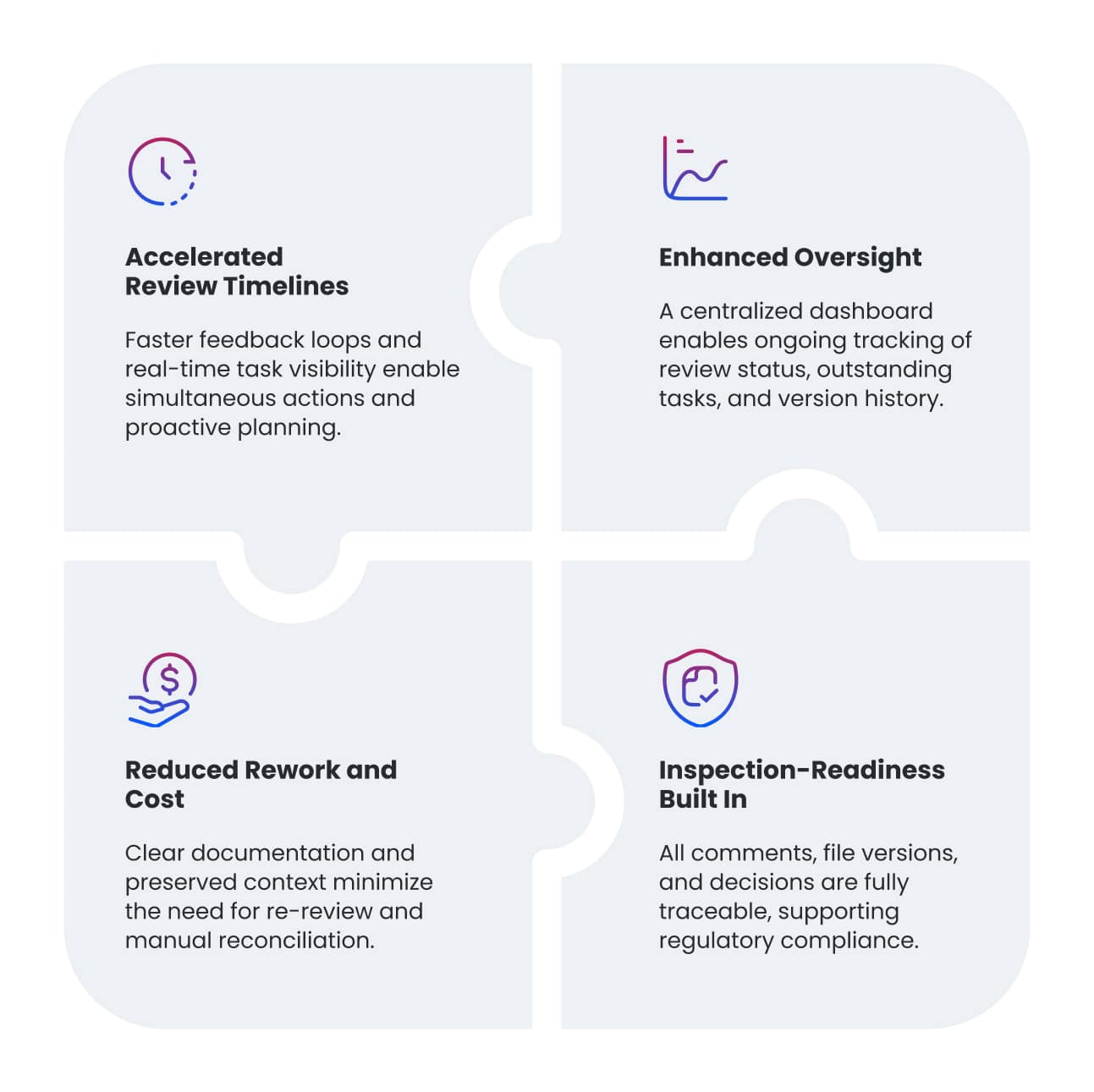
Taken together, these benefits enable faster decision-making, shorter development timelines, and quicker progression to critical milestones—delivering cost savings and meaningful acceleration in trial execution and team efficiency.
Efficient, Real-Time Review in Practice:
Compressing Review Timelines to Deliver Therapies to Patients Faster
In a recent Phase III study for a global top 10 pharma sponsor, Phastar used Verify to manage the review of hundreds of files at the Final Analysis stage prior to sponsor handoff. The reviewers — statisticians—created various review tasks and assigned them to the lead statistical programmer for discussion and resolution.
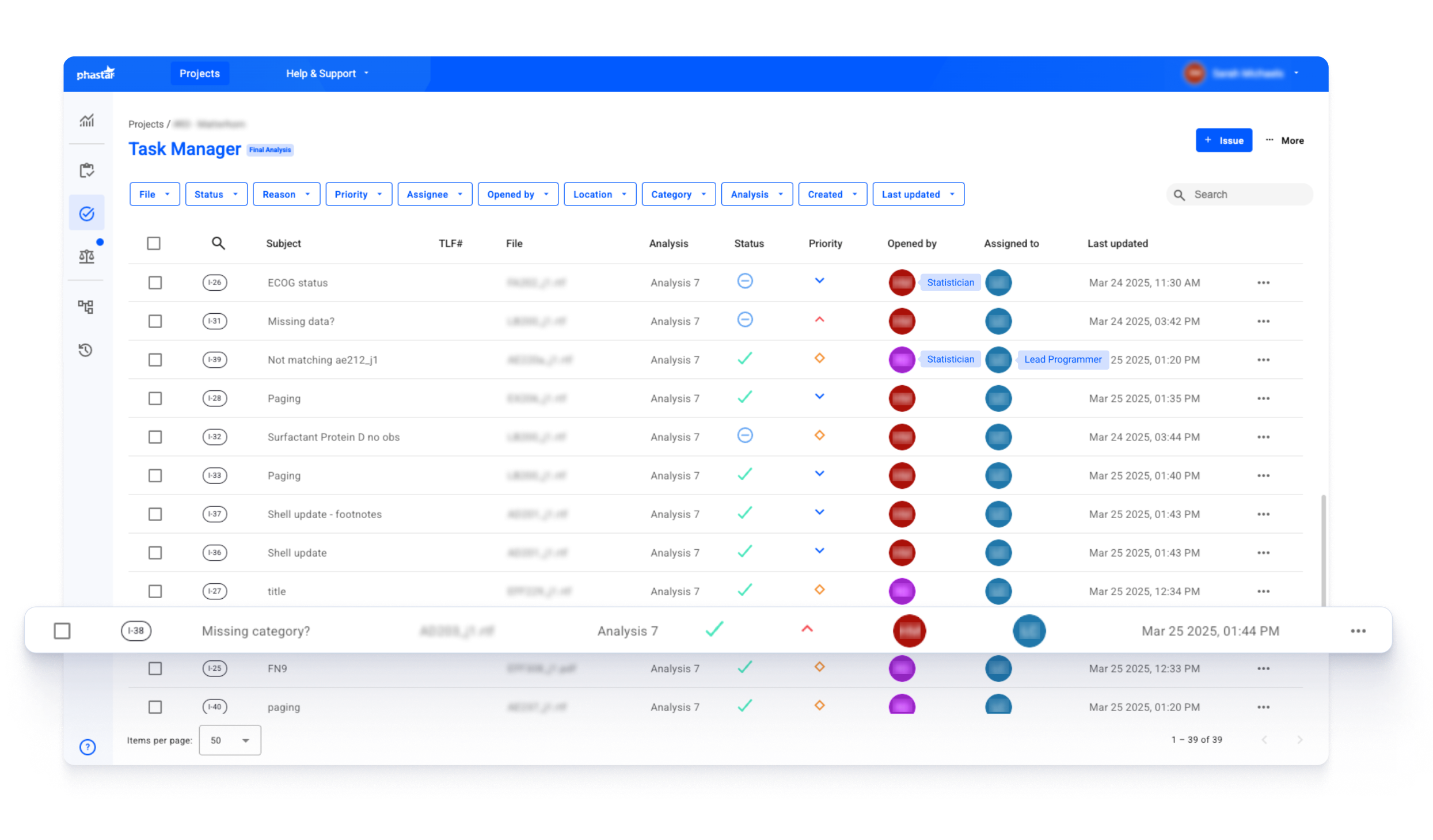
The Task Manager view within Verify allows stakeholders to gain immediate visibility into project tasks, including status, priority, and assignment. In addition to Phastar reviewers, sponsor reviewer roles include sponsor study team members, statisticians, programmers, data managers, and medical writers—enabling coordinated communication across functions and organizations.
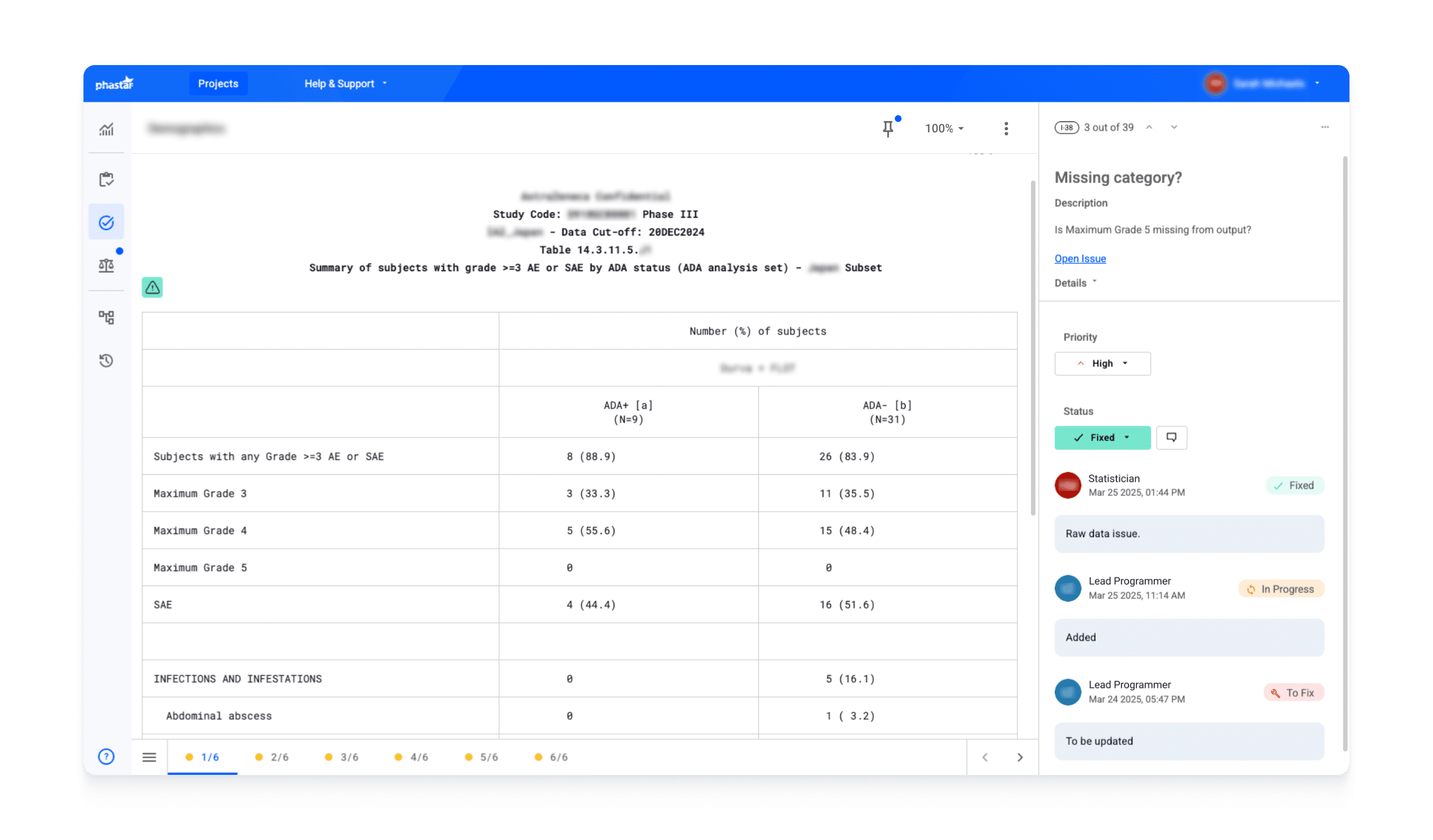
Each file review task within Verify enables context-specific communication directly on digitized output files, without the need for additional trackers or annotated PDFs. This allows a traceable, real-time interactions about output structure, data, versions, management tasks, and any other submission-related issues, with the full history automatically preserved.
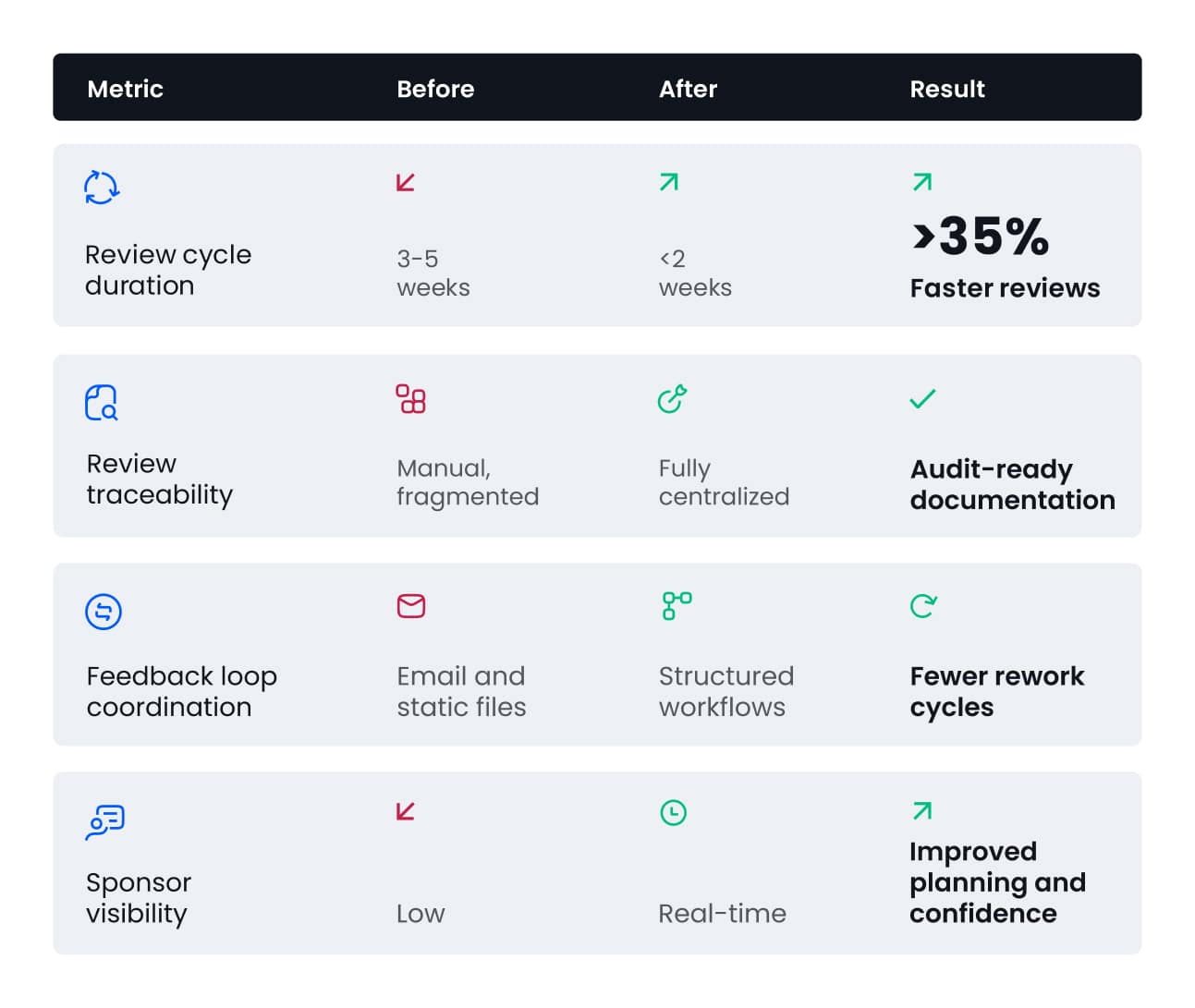
With this centralized, transparent approach, Phastar has reduced review cycle times from 3-5 weeks to less than 2 weeks, achieving at least 35% reduction in average clinical data review timelines – supporting accelerated time to submission and faster delivery of therapies.
The Impact:
Faster Reviews, Enhanced Oversight, Measurable Results
Using the Verify platform has transformed Phastar’s review operations, demonstrating measurable impact across live studies:
Conclusion:
Building Data Review Processes for the Future
The collaboration between Beaconcure and Phastar illustrates a broader movement across clinical data review: rethinking how we efficiently manage data oversight, documentation, and traceable communication.
With increasing trial complexity and growing regulatory expectations, there’s an urgent need to replace fragmented processes with systems that are better suited to today’s clinical landscape, and offer transparency, accountability, and adaptability.
The Verify platform—and a robust partnership between technology providers and specialist biometrics and data science experts—play a key role in that transformation.
